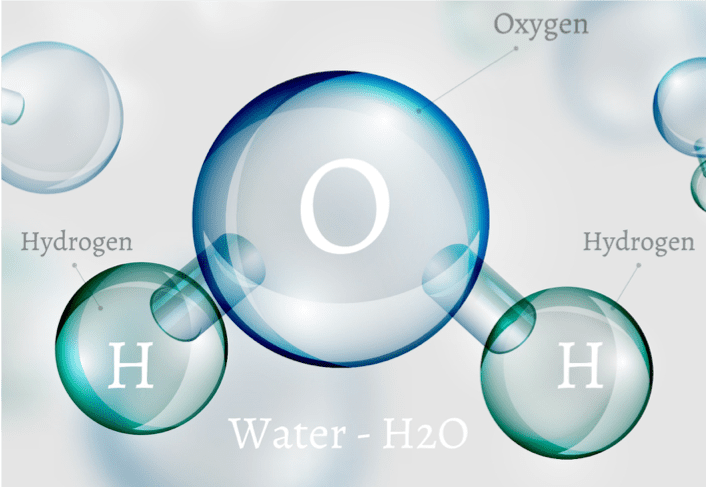
The H2O Molecule (water) is composed of:
- 1 Oxygen atom “O” (electro-negative charge)
- Nucleus composed by 8 protons (positive electrical charge) & 8 neutrons
- 8 electrons (negative electric charge) rotating on themselves and orbiting the atom O (spin) = electronic cloud
- 2 Hydrogen atoms “H” (electro-positive charge)
- Nucleus composed by 1 proton (positive electrical charge)
- 1 electron (negative electric charge) rotating on itself and orbiting each atom H (spin) = electronic cloud
The H2O Molecule is a dipole because there is a potential difference between the O atom (negative charge) & the 2 H atoms (positive charge) which make a covalent bond between them (the 2 nuclei share their electrons). In fact, energy flows from one pole to another and its path is visualized in the structure of water. The water molecule also makes non-covalent bonds by hydrogen bridges between water molecules and molecular bonds between H2O and minerals, pollutants, etc.
As long as it is liquid, the water molecule is rotating around its center of mass (spin)
The diameter of the H2O molecule (between the 2 hydrogen atoms) is 1.38 Angströms = 0.138 nm (nanometers)