Analysis carried out according to international standards in July 2021 by the SGS laboratory in Rotterdam, the Netherlands (Krüss Easy Dyne device). The ring tear-off method measures the surface tension of water: The platinum ring is submerged and then pulled upward until it crosses the surface of the liquid. The lamella is overstretched until it breaks and gives the measure of the surface tension of the water.
Comparison of the surface tension of dynamized and non-dynamized tap water from Rhode Saint Genèse (Belgium)
After analysis, it turns out that the water dynamized by the Biodynamizer has a lower surface tension of -15% compared to non-dynamized water (66 dynes / cm -> 56 Dynes / cm or mN / m at 20 °C and measured according to ASTM D1331).
Knowing that a lower surface tension has a “more wetting” power which optimizes the penetration of water into the cells and therefore allows better cellular hydration (this occurs via aquaporins through which +/- 1 billion molecules water enter the cell one by one every second).
This better hydration would help to eliminate toxins and metabolic waste and therefore detoxify the body.
“If nutrients cannot enter the cells because of the high surface tension of the water, then the cells dehydrate and die from the accumulation of their own wastes” Nobel Prize winner Alexis Carrel.
This cellular detoxification is therefore amplified by:
- A lower surface tension of water (because the water then penetrates better into the cells via aquaporins),
- A modification of the membrane voltage of the cell which activates the ion channels (water with an electronegative charge promotes the negative potential of the membrane and so the opening/closing of the ion channels)
This drop in surface tension of dynamized water was also observed by:
- electrophotonic analysis (spreading of the dynamized water drop)
- studies acquired on the influence of magnetism on water


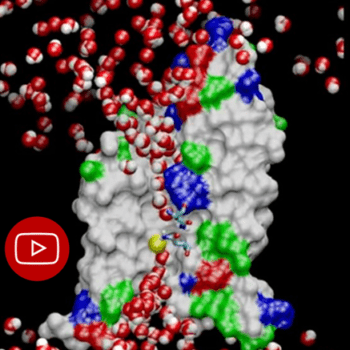
Aquaporins let +/- 1 billion water molecules pass one after the other into the cell every second! (Visualized on the video by the small yellow molecule); The surface tension of the water plays an important role in this process!
Copyright NIH Center for Macromolecular Modeling and Bioinformatics at the Beckman Institute, University of Illinois (http://www.ks.uiuc.edu/)